Which of the Following Has No Net Dipole Moment
A molecule which has no net dipole moment is called. But zero dipole moment is not only due to non polar bonds of zero electronegative difference.
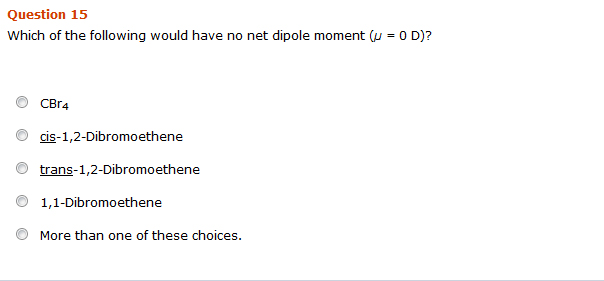
Solved Which Of The Following Would Have No Net Dipole Chegg Com
Andwhya H2O bNF3 cH2Se dTeO3 e CH3Cl2-.

. The structure of compounds given in options are as follows. The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in the molecule. Such is the case for CO 2 a linear molecule part a in Figure 228.
Chemistry questions and answers. Which of the following has no net dipole moment. So according to this the correct option is d.
2 CCIA 3 BeF24 SO Open in App. 101 Which of the following has no net dipole moment. The normal boiling point for H2Te is higher.
CsBrg CCl2F2g H2g CO2g CsBrg Which of the following molecules does not have a dipole moment. Which of the following should have the largest dipole moment. What is a non-recording rain-gauge.
Few compounds are Polar to but have zero dipole moment because they have symmetry. Dipole moments occur when there is a separation of charge. Start your trial now.
Weve got the study and writing resources you need for your assignments. A Gases B Vaccum C Liquids D Solids. We review their content and use your feedback to keep the quality high.
Thus CCl 4 has no dipole moment. This is because the individual dipole moments cancel out because of the symmetrical tetrahedral shape of the molecule. A SCl2 B H2O C CF4 D BrCl.
Predict the ideal bond angles in FNO using the molecular shape given. Which of the following has a net dipole moment. In non-polar molecule the centres of positive and negative charges coincide.
Answer 1 of 7. A BeCl2 B CCl4 C CO2 D SF2. Which of the following molecules has a net dipole moment.
A molecule which has no net dipole moment is called non-polar. Each CO bond in CO 2 is polar yet experiments show that. Solution for Which of the following has no net dipole moment.
Zero dipole moment include all non polar molecules like H2 N2 O2 Cl2 etc. N 2 O NF 3 H 2 Se TeO 3 CH 3 Cl. Experts are tested by Chegg as specialists in their subject area.
A BF3 B NCl3 C H2Se D CH3Cl. If the individual bond dipole moments cancel one another there is no net dipole moment. Speed of sound is maximum in which among the following.
A molecule which has a symmetrical geometry will have no dipole moment as the magnitude of all the bond moments cancel each other. Which of the following does not have a molecular dipole moment. Which of the following molecules has polar bonds but has no net dipole.
1-Which of the following has no net dipole moment. 95 130 ratings play-rounded-fill. Which of the below molecules has no net dipole moment.
Among all the given options TeO3 has no net dipole moment because the central atom has three bonded atoms one bond being a double bond and has no lone pairs and the geometry is trigonal planar with symmetrical distribution of dipoles resulting in dipole cancellations and has no net dipole moment. PH3 HBr CH3OH CH3CH3. Carbon tetrachloride molecule has zero dipole moment even though C and Cl have different electronegativities and each of the C - Cl bond is polar and has some dipole moment.
First week only 499. Why does CCl4 have no dipole moment. This occurs due to an atoms electronegativity - where one atom has the ability to attract electrons towards it In other words electrons wants to spend other time around it giving it a negative charge and the other a positive charge.
Net dipole operates on the same idea - but it focuses on the direction and. O CH CI NF3 N20 O TeO3 H2Se O O O. Who are the experts.
The study of diple moment of a molecule is useful to explain the shape of a molecule and also to predict a The net dipole moment of a polyatomic molec asked Sep 26 in Chemistry by Arnika Singh 736k points. What is zero.

Solved 1 Which Of The Following Has No Net Dipole Moment O Chegg Com

Answered Which Of The Following Has No Net Bartleby

Solved Which Of The Following Has No Net Dipole Moment A Chegg Com

Which Of The Following Alkanes Has No Net Dipole Moment Youtube
No comments for "Which of the Following Has No Net Dipole Moment"
Post a Comment